Convenient synthesis of pentafluoroethyl thioethers via catalytic Sandmeyer reaction with a stable fluoroalkylthiolation reagent†
Abstract
Aromatic and heteroaromatic diazonium salts were smoothly converted into the corresponding pentafluoroethyl thioethers by reaction with Me4NSC2F5 in the presence of catalytic amounts of elemental copper. This Sandmeyer-type reaction proceeds at room temperature under mild conditions and is applicable to a wide range of functionalised molecules. It enables the late-stage introduction of pentafluoroethylthio groups, a promising but largely unexplored substituent, into bioactive molecules.
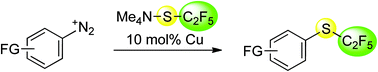
- This article is part of the themed collection: Celebrating the 75th Birthday of Professor Barry Trost

 Please wait while we load your content...
Please wait while we load your content...