Modulating single-molecule magnet behaviour of phenoxo-O bridged lanthanide(iii) dinuclear complexes by using different β-diketonate coligands†
Abstract
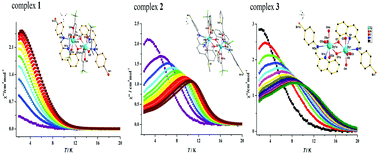
Three dinuclear Dy(III) complexes, [Dy2(tfa)4L2] (1), [Dy2(TTA)4L2] (2) and [Dy2(dbm)4L2·2CH3CN·0.5H2O] (3) (tfa = trifluoroacetylacetonate, TTA = 2-thenoyltrifluoroacetone, dbm = dibenzoylmethane and HL = 2-[((4-bromophenyl)-imino)methyl]-8-hydroxyquinoline, have been synthesized, and structurally and magnetically characterized. The Dy(III) ions are eight-coordinated with two bidentate β-diketonate and two μ2-O bridging 8-hydroxyquinoline Schiff base ligands. Magnetic studies reveal that 1–3 exhibit different magnetic relaxation behaviors with the anisotropic barriers of 9.61 K (1), 54.81 K (2) and 30.98 K (3), respectively. The different magnetic relaxation behaviors of the three Dy2 complexes originate from the different chemical environments of the central Dy3+ ions with different β-diketonate coligands.

 Please wait while we load your content...
Please wait while we load your content...