Singlet oxygen photosensitisation by the fluorescent protein Pp2FbFP L30M, a novel derivative of Pseudomonas putida flavin-binding Pp2FbFP†‡
Abstract
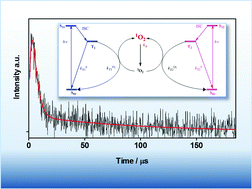
Flavin-binding fluorescent proteins (FbFPs) are a class of fluorescent reporters that have been increasingly used as reporters in the study of cellular structures and dynamics. Flavin's intrinsic high singlet oxygen (1O2) quantum yield (ΦΔ = 0.51) provides a basis for the development of new FbFP mutants capable of photosensitising 1O2 for mechanistic and therapeutic applications, as recently exemplified by the FbFP miniSOG. In the present work we report an investigation on the 1O2 photoproduction by Pp2FbFP L30M, a novel derivative of Pseudomonas putida Pp2FbFP. Direct detection of 1O2 through its phosphorescence at 1275 nm yielded the value ΦΔ = 0.09 ± 0.01, which is the highest 1O2 quantum yield reported to date for any FP and is approximately 3-fold higher than the ΦΔ for miniSOG. Unlike miniSOG, transient absorption measurements revealed the existence of two independent triplet states each with a different ability to sensitise 1O2.
- This article is part of the themed collection: Photofunction Proteins


 Please wait while we load your content...
Please wait while we load your content...