Photocatalytic fluoroalkylation reactions of organic compounds
Abstract
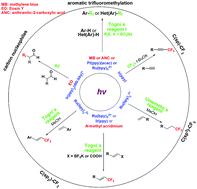
Photocatalytic methods for fluoroalkyl-radical generation provide more convenient alternatives to the classical perfluoroalkyl-radical (Rf) production through chemical initiators, such as azo or peroxide compounds or the employment of transition metals through a thermal electron transfer (ET) initiation process. The mild photocatalytic reaction conditions tolerate a variety of functional groups and, thus, are handy to the late-stage modification of bioactive molecules. Transition metal-photocatalytic reactions for Rf radical generation profit from the redox properties of coordinatively saturated Ru or Ir organocomplexes to act as both electron donor and reductive species, thus allowing for the utilization of electron accepting and donating fluoroalkylating agents for Rf radical production. On the other hand, laboratory-available and inexpensive photoorgano catalysts (POC), in the absence of transition metals, can also act as electron exchange species upon excitation, resulting in ET reactions that produce Rf radicals. In this work, a critical account of transition metal and transition metal-free Rf radical production will be described with photoorgano catalysts, studying classical examples and the most recent investigations in the field.

 Please wait while we load your content...
Please wait while we load your content...