Copper-catalysed azide–alkyne cycloadditions (CuAAC): an update
Abstract
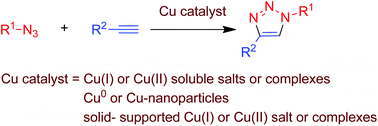
The reactions of organic azides and alkynes catalysed by copper species represent the prototypical examples of click chemistry. The so-called CuAAC reaction (copper-catalysed azide–alkyne cycloaddition), discovered in 2002, has been expanded since then to become an excellent tool in organic synthesis. In this contribution the recent results described in the literature since 2010 are reviewed, classified according to the nature of the catalyst precursor: copper(I) or copper(II) salts or complexes, metallic or nano-particulated copper and several solid-supported copper systems.

 Please wait while we load your content...
Please wait while we load your content...