Morita–Baylis–Hillman adduct derivatives (MBHADs): versatile reactivity in Lewis base-promoted annulation
Abstract
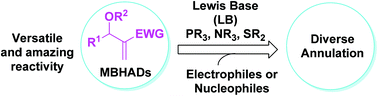
Lewis base-promoted annulation reactions with MBHADs have emerged as a key platform for the construction of functionalized carbo- and heterocycles. MBHADs, which are economical and readily available, exert diverse and amazing reactivity when reacted with a wide range of electrophiles. A variety of carbo- and heterocycles, most of which are predominant in natural products and pharmaceuticals, could be constructed with high efficiency. This tutorial review will describe these annulation reactions, with a special emphasis on recent work regarding diverse reactivities of MBHADs.

 Please wait while we load your content...
Please wait while we load your content...