Iron- or silver-catalyzed oxidative fluorination of cyclopropanols for the synthesis of β-fluoroketones†
Abstract
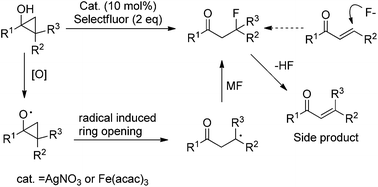
The FeIII- or AgI-catalyzed oxidative fluorination of cyclopropanols via radical rearrangement is disclosed. This process features a straightforward and highly effective protocol for the site-specific synthesis of β-fluoroketones and represents an expedient method for the synthesis of γ-, δ- and ε-fluoroketones. Notably, this reaction proceeds at room temperature and tolerates a diverse array of cyclopropanols.

 Please wait while we load your content...
Please wait while we load your content...