O-Glycosylation methods in the total synthesis of complex natural glycosides
Abstract
Covering: 1994–2015
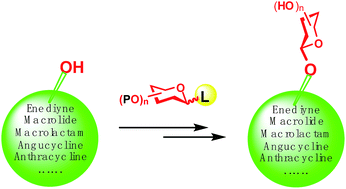
The total syntheses of 33 complex natural O-glycosides, such as the glycosides of macrocyclic lactones/lactams, enediynes, angucyclines, and anthracyclines, are highlighted, with a major focus being placed on the O-glycosylation reactions which connect the saccharides and the aglycones. These successful O-glycosylation reactions employ such donors as glycosyl bromides, fluorides, iodides, trichloroacetimidates, N-phenyl trifluoroacetimidates, thioglycosides, sulfoxides, heteroaryl thioglycosides, 1-hydroxyl sugars, 1-O-acetates, and ortho-alkynylbenzoates. Each synthesis is depicted starting from the O-glycosylation of the aglycone (or its precursor); the glycosylation conditions and outcomes (yields and stereoselectivities) are discussed, and the subsequent transformations toward the final target, including the elongation of the glycan, the elaboration of the aglycone, and the protecting group manipulations are also given in detail.

 Please wait while we load your content...
Please wait while we load your content...