One-pot, efficient and green synthesis of acridinedione derivatives using highly monodisperse platinum nanoparticles supported with reduced graphene oxide†
Abstract
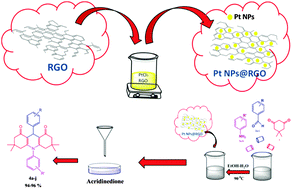
An efficient, high yielding, quick method has been developed for the synthesis of acridinedione derivatives via a one-pot, multi-component condensation of dimedone (1), various aromatic aldehydes (2a–i), and various aromatic amines (3a,b) using highly monodisperse platinum nanoparticles supported with reduced graphene oxide (Pt NPs@rGO) as a recyclable heterogeneous catalyst. This method takes advantage of the fact that water/ethanol (a green solvent) is used in combination with Pt NPs@rGO nanoparticles as a catalyst which can be easily recovered and reused for further runs. This highly monodisperse catalyst has been used for the first time as a highly stable, exceptionally reusable, isolable, bottleable, long-lived and highly efficient catalyst for the synthesis of acridinedione derivatives from dimedone, aromatic aldehydes and various amines with great catalytic performance. The synthesized catalyst was characterized using transmission electron microscopy, high resolution electron microscopy, X-ray diffraction, Raman and X-ray photoelectron spectroscopy techniques. The results of these analyses show the formation of highly crystalline and colloidally stable highly monodisperse Pt NPs@rGO. This highly monodisperse catalyst is a most efficient catalyst which gives the high yield of products in a short time.

 Please wait while we load your content...
Please wait while we load your content...