Synthesis, characterization and reactivity of a six-membered cyclic glycerol carbonate bearing a free hydroxyl group†
Abstract
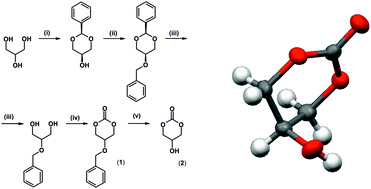
Five- and six-membered cyclic carbonates have recently become popular as starting materials for the synthesis of polycarbonates via ring opening polymerization or synthesis of environmentally friendly non-isocyanate polyurethanes. In many cases a five-membered glycerol carbonate has been used in these applications. However, the simplest derivative of glycerol, a six-membered cyclic glycerol carbonate (5-hydroxy-1,3-dioxan-2-one), has not been reported so far. In this work, for the first time, we report a procedure for the synthesis of this monomer from glycerol. The product was characterized by 1H NMR, 13C NMR, and FTIR spectroscopy and X-ray diffraction measurements. Further, the synthesis of bis(2-oxo-1,3-dioxan-5-yl) sebacate, a biscyclic six-membered carbonate, was described. The reactivities of 5-hydroxy-1,3-dioxan-2-one and its biscyclic ester derivative were investigated. No ring opening polymerization of both the monomers was observed, instead an isomerization to appropriate five-membered cyclic carbonates occurred. Unfortunately, the protection of the hydroxyl group 2 with an ester type substituent does not protect it against isomerisation.

 Please wait while we load your content...
Please wait while we load your content...