A shock tube study of the branching ratios of propene + OH reaction
Abstract
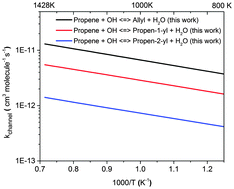
Absolute rate coefficients for the reaction of the OH radical with propene (C3H6) and five deuterated isotopes, propene-1-D1 (CDHCHCH3), propene-1,1-D2 (CD2CHCH3), propene-1,1,2-D3 (CD2CDCH3), propene-3,3,3-D3 (CH2CHCD3), and propene-D6 (C3D6), were measured behind reflected shock waves over the temperature range of 818–1460 K and pressures near 1 atm. The reaction progress was followed by monitoring the OH radical near 306.7 nm using UV laser absorption. Kinetic isotope effects in the measured rate coefficients are discussed and rationalized for the site-specific H-abstraction by the OH radical. The first experimental measurements for the branching ratio of the title reaction are reported and compared with transition state theory calculations. The allylic H-atom abstraction of propene by OH radicals was found to be the most dominant reaction pathway followed by propen-1-yl and propen-2-yl channels over the entire temperature range of this study. The derived Arrhenius expressions for various site-specific rate coefficients over 818–1442 K are (the subscript in the rate coefficient identifies the position of H or D atom according to the IUPAC nomenclature of alkenes):

 Please wait while we load your content...
Please wait while we load your content...