Room temperature spontaneous conversion of OCS to CO2 on the anatase TiO2 surface†
Abstract
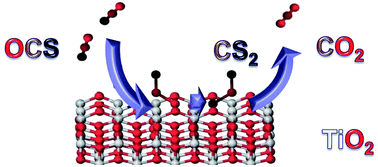
High-resolution FT-IR spectroscopy combined with quantum chemical calculations was used to study the chemistry of OCS-disproportionation over the reduced surface of isotopically labelled, nanocrystalline TiO2. Analysis of the isotopic composition of the product gases has revealed that the reaction involves solely OCS molecules from the gas-phase. Using quantum chemical calculations we propose a plausible mechanistic scenario, in which two reduced Ti3+ centres mediate the reaction of the adsorbed OCS molecules.

 Please wait while we load your content...
Please wait while we load your content...