Water-based synthesis and cleaning methods for high purity ZnO nanoparticles – comparing acetate, chloride, sulphate and nitrate zinc salt precursors†
Abstract
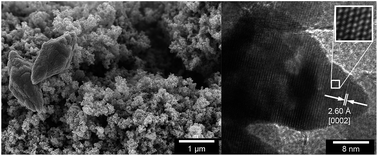
A low temperature (60 °C) aqueous synthesis method of high purity ZnO nanoparticles intended as fillers for ultra-low electrical conductivity insulations is described. Particles were prepared under identical conditions from different zinc salts based on nitrate, chloride, sulphate or acetate to compare their abilities to form high yields of sub-50 nm particles with narrow size distribution. The acetate salt gave uniform 25 nm ZnO particles with a conical prism shape. The chloride and sulphate derived particles showed mixed morphologies of nanoprisms and submicron petals, whereas the nitrate salt yielded prisms assembled into well-defined flower shapes with spiky edges. The micron-sized flower shapes were confirmed by X-ray diffraction to consist of the smaller prism units. Photoluminescence spectroscopy showed emission in the blue-violet region with little variation depending on precursor salt, suggesting that the spectra were dependent on the primary nanoprism formation and rather independent of the final particle morphology. Microscopy revealed that the salt residuals after the reaction showed different affinity to the particle surfaces depending on the type of salt used, with the acetate creating ca. 20 nm thick hydrated shells; and in falling order of affinity: chloride, sulphate and nitrate. An acetate ion shielding effect during the synthesis was therefore assumed, preventing nanoparticle fusion during growth. Varying the concentrations of the counter-ions confirmed the shielding and only the acetate anions showed an ability to stabilize solitary nanoprisms formation in reaction yields from 2 to 10 g L−1. Ultrasonic particle surface cleaning was significantly more efficient than water replacement, resulting in a stable aqueous dispersion with a high zeta potential of 38.9 mV at pH 8.

 Please wait while we load your content...
Please wait while we load your content...