Adsorption mechanism of Ca2+, Mg2+, Fe3+, and Al3+ ions in phosphoric acid–nitric acid solution on 001 × 7 and S957 resins
Abstract
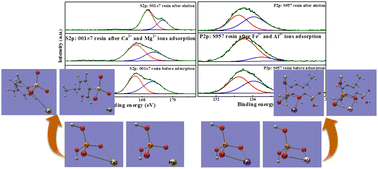
Selective removal of Ca2+ and Mg2+ ions using the 001 × 7 resin and Fe3+ and Al3+ ions using the S957 resin is able to achieve the deep purification of the phosphoric acid–nitric acid solution, but the adsorption behaviors of Fe3+ and Al3+ ions are seriously suppressed by phosphoric acid. In order to understand the interaction mechanism of separation processes and the influence of phosphoric acid, we first studied the bonding form of Ca2+, Mg2+, Fe3+, and Al3+ ions on 001 × 7 and S957 resins using FT-IR and XPS techniques; subsequently, quantum chemistry computation was carried out to further explore the bonding mechanism between the functional groups on resins and metal ions. FT-IR and XPS results reveal that for the adsorption process on the 001 × 7 resin, hydroxyls from sulfonic acid groups combine with Ca2+ and Mg2+ ions. Whereas Fe3+ and Al3+ ions are adsorbed on the S957 resin through an exchange reaction with hydroxyls on the phosphonic acid group but not on the sulfonic acid group. Quantum chemistry computation results reveal that the phosphonic acid group has a larger binding energy with Fe3+ and Al3+ ions. Thus, the S957 resin still presents great adsorption performance for Fe3+ and Al3+ ions despite the influence of dihydrogen phosphate ions in the phosphoric acid–nitric acid solution.


 Please wait while we load your content...
Please wait while we load your content...