Use of benzothiophene ring to improve the photovoltaic efficacy of cyanopyridinone-based organic chromophores: a DFT study†
Abstract
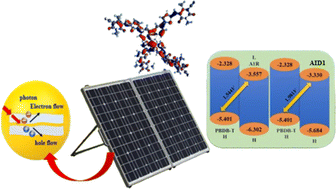
The benzothiophene based chromophores (A1D1–A1D5) with A–π–A configuration were designed via end-capped tailoring with benzothiophene type acceptors using reference compound (A1R). Quantum chemical calculations were accomplished at M06/6-311G(d,p) level to probe optoelectronic and photophysical properties of designed chromophores. Therefore, frontier molecular orbitals (FMOs), binding energy (Eb), open circuit voltage (Voc), transition density matrix (TDM), density of state (DOS) and UV-Vis analyses of A1R and A1D1–A1D5 were accomplished. The designed compounds (A1D1–A1D5) exhibited absorption values in the visible region as 616.316–649.676 nm and 639.753–665.508 nm in gas and chloroform phase, respectively, comparing with reference chromophore. An efficient charge transference from HOMO towards LUMO was found in A1D1–A1D5 chromophores which was further supported by TDM and DOS analyses. Among all chromophores, A1D2 exhibited unique characteristics such as reduced band gap (2.354 eV), higher softness (σ = 0.424 eV), lower exciton binding energy (0.491 eV) and maximum value of open circuit voltage (Voc = 1.981 V). Consequently, A1D2 may be considered as potential candidate for the development of optoelectronic devices. These analyses revealed that the studied compounds exhibited promising findings. They may be utilized in the realm of organic solar cells.


 Please wait while we load your content...
Please wait while we load your content...