Efficient ambient ammonia synthesis by Lewis acid pair over cobalt single atom catalyst with suppressed proton reduction†
Abstract
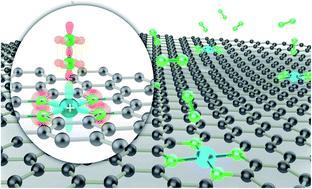
Improving the ammonia yield and Faraday efficiency of ambient electrochemical nitrogen fixation is a priority for altering the energy-intensive Haber–Bosch process. In this work, positively charged single cobalt atoms anchored on sponge-like nitrogen-doped mesoporous interconnected hollow carbon nanofibers (serving as a Lewis acid pair) were intentionally designed as catalytic centers that can suppress the side effect of the competing hydrogen evolution reaction and simultaneously boost the electrochemical conversion of nitrogen (N2) to ammonia (NH3). The Lewis acid pair catalyst exhibits an NH3 production rate of 67.6 μg h−1 mg−1 and a maximum Faraday efficiency of 56.9% at a peak potential of −0.1 V vs. RHE, which outperforms previously reported nitrogen reduction reaction (NRR) catalysts. First-principles DFT calculations suggest the regulation of the local electronic structure that induces Lewis acid pair formation upon charge transfer between the single Co atom and substrate, confirming a high intrinsic NRR by both experiments and theoretical calculations.


 Please wait while we load your content...
Please wait while we load your content...