Iodolium salts as halogen-bond donor catalysts in the Nazarov cyclization: the molecular oxygen enigma†
Abstract
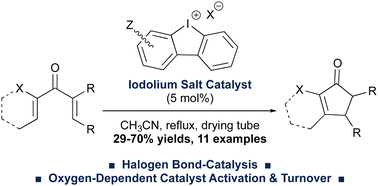
Cyclic diaryliodonium (e.g. iodolium) salts are effective Lewis-acidic halogen-bond donor catalysts of the Nazarov cyclization. The reaction worked across a series of variously-substituted, activated divinyl ketone precursors, giving the cyclopentenone products in up to 70% isolated yield. Control experiments suggested that air or oxygen were required for catalyst activation and turnover, which constitutes a new, albeit enigmatic, phenomenon in diaryliodonium salt catalysis.


 Please wait while we load your content...
Please wait while we load your content...