Asymmetric hydroamination with far fewer chiral species than copper centers achieved by tuning the structure of supramolecular helical catalysts†
Abstract
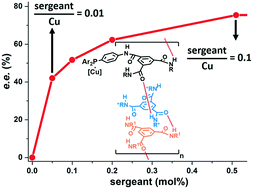
The incorporation of a few chiral monomers (the “sergeants”) in a backbone composed of a majority of achiral monomers (the “soldiers”) is a well-established strategy to control the handedness of helical polymers. However, the implementation of this “sergeants-and-soldiers” effect in asymmetric catalysis is still at its infancy, with only limited examples for which the sergeant amount is actually in lower amount than the (metal) catalytic unit. Herein, supramolecular co-polymers composed of a benzene-1,3,5-tricarboxamide (BTA) phosphine soldier and a catalytically-inactive BTA sergeant were evaluated in the copper-catalysed hydroamination of styrene. Screening of various BTA ligands revealed the marked influence of substituents on the aryl group of the BTA phosphine ligand, the 3,5-bis-CF3-substituted ligand providing the highest rate and enantioselectivity. Thorough optimization of the reaction parameters led to a robust protocol for the generation of the amine product in high yield (82 ± 4%) and moderate e.e. (68 ± 6%). Addition of an achiral BTA additive was found to be beneficial for improving the yield (80–99%), enantioselectivity (up to 81% e.e.) and “sergeants-and-soldiers” effect displayed by the supramolecular helical catalyst. Consequently, an enantio-enriched product (75% e.e.) was afforded with as low as 0.51 mol% of sergeant in the catalytic mixture, i.e. one chiral molecule for 10 copper centers.
- This article is part of the themed collection: Emerging Investigator Series


 Please wait while we load your content...
Please wait while we load your content...