Organocatalyst based cross-catalytic system†
Abstract
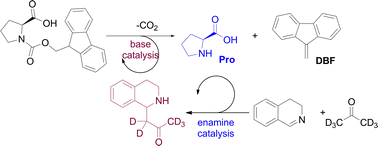
We present our design of a cross-catalytic system based on organocatalysis. The system features two organic reactions, namely a deprotection reaction of Fmoc protected proline and a Mannich reaction between acetone and dihydroisoquinoline. The products of these two reactions, proline and a tetrahydroisoquinoline, respectively, are capable of reciprocal reaction rate enhancement. Detailed kinetic studies of the system and seeding experiments support the cross-catalytic relationship in the reaction network.
- This article is part of the themed collections: Celebrating 10 years of ChemComm Emerging Investigators and Chemical Communications HOT Articles 2022


 Please wait while we load your content...
Please wait while we load your content...