Decomposition pathways and mitigation strategies for highly-stable hydroxyphenazine flow battery anolytes†
Abstract
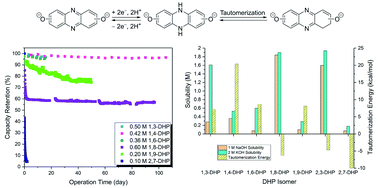
Aqueous organic redox flow batteries are a promising technology for large-scale energy storage. The stability of the redox active organic molecules is increasingly being recognized as one of the major hurdles. Upon extended flow battery cycling, 7,8-dihydroxyphenazine-2 sulfonic acid (DHPS) undergoes desulfonation and reduction of a phenolic C–O bond to yield a mixture of 7/8-hydroxyphenazine-2-sulfonic acid, as well as hydrogenation of the aromatic ring system. Density functional theory (DFT) analysis of the charged DHPS, its ring-hydrogenated products, and variably substituted hydroxy phenazines has led to the development of a series of dihydroxylated phenazine isomers which provide insight into the effects of substitution pattern on solubility and stability. Seven dihydroxyphenazine (DHP) isomers were synthesized and their solubilities, electrochemical properties, flow battery cycling performance, and degradation pathways have been investigated. Based on theoretical and experimental results, hydroxyl substitution at the 1, 4, 6 and 9 positions yields highly stable derivatives, while substitution at the 2, 3, 7, and 8 positions results in unstable derivatives. Flow cells of 1,4- and 1,6-DHPs coupled with ferro/ferricyanide achieved high stabilities, with temporal capacity loss of 0.029 and 0.031% per day, respectively. Decomposition of 1,8- and 2,7-DHPs were found to arise from irreversible hydrogen rearrangement (tautomerization), yielding redox-inactive species. These results provide a detailed understanding of decomposition pathways and mitigation strategies for phenazine-based anolytes, and can provide general design guidelines for the development of stable redox-active organics.


 Please wait while we load your content...
Please wait while we load your content...