Combining palladium and ammonium halide catalysts for Morita–Baylis–Hillman carbonates of methyl vinyl ketone: from 1,4-carbodipoles to ion pairs†
Abstract
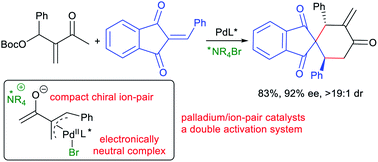
Here we report that Morita–Baylis–Hillman carbonates from diverse aldehydes and methyl vinyl ketones can be directly utilised as palladium-trimethylenemethane 1,4-carbodipole-type precursors, and both reactivity and enantioselectivity are finely regulated by adding a chiral ammonium halide as the ion-pair catalyst. The newly assembled intermediates, proposed to contain an electronically neutral π-allylpalladium halide complex and a reactive compact ion pair, efficiently undergo asymmetric [4 + 2] annulations with diverse activated alkenes or isatins, generally with high regio-, diastereo- and enantio-selectivity, and even switchable regiodivergent or diastereodivergent annulations can be well realised by tuning the substrate or catalyst assemblies. An array of control experiments, including UV/Vis absorption study and density functional theory calculations, are conducted to rationalise this new double activation mode combining a palladium complex and an ammonium halide as an ion-pair catalyst.


 Please wait while we load your content...
Please wait while we load your content...