Activator free diastereoselective 1,3-dipolar cycloaddition: a quick access to coumarin based spiro multi heterocyclic adducts†
Abstract
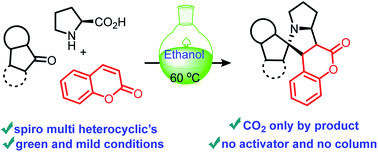
A formal diastereoselective 1,3-dipolar cycloaddition of azomethine ylide and coumarin derivatives to construct coumarin based spiro multi heterocyclics has been described. The in situ generation of azo-ylide was achieved for various heterocyclic carbonyls (indenoquinoxaline and isatin). This transformation is also suitable for maleimide dipolarophiles for the synthesis of hydro-maleimide derivatives. These decarboxylative annulations neither required any catalyst nor any activator. Further the pure products were isolated by filtration from the reaction mixture after the reaction under ambient conditions.


 Please wait while we load your content...
Please wait while we load your content...