Revisiting the valence stability and preparation of perovskite structure type oxides ABO3 with the use of Madelung electrostatic potential energy and lattice site potential
Abstract
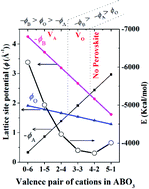
Valence stability of aliovalent ions is mostly correlated with lattice site potential in ionic crystals. Madelung electrostatic potential is obtained by adding all the lattice site potentials for all the ions present in a crystal structure. Therefore, valence stability and the stability of a crystal structure can be better understood with consideration of both the lattice site potential and Madelung electrostatic potential. This was first demonstrated more than four decades ago by one of the present authors. We revisit this situation by using re-calculated lattice site potential and Madelung electrostatic potential for perovskite structure type ABO3 compounds using a new computer program VESTA. We show that the formation of a perovskite structure type compound with the general formula ABO3 (where A and B are cations and O is an oxide ion) becomes energetically favorable when it has a higher Madelung electrostatic potential than the combined Madelung electrostatic potential of parent binary compounds AO and B2O3 or BO2. It is further shown that strong lattice site potential results in stability of high valence or high valence ions can be stabilized in a lattice site with strong lattice-site potential. It further follows that certain ions experience maximum lattice site potential at the B ion lattice site of the perovskite structure when compared to other structures such as fluorite BO2, rutile BO2 and corundum B2O3. Therefore, (i) the stability of an ion with a high (and uncommon) valence state at the B site being higher than that at the A site, (ii) occurrence of point defects at A or O sites with weak lattice site potentials, respectively and (iii) instability of perovskite A4+B2+O3, and A5+B1+O3 compounds, respectively can be rationalized by lattice site potential and Madelung electrostatic potential analysis.


 Please wait while we load your content...
Please wait while we load your content...