Hydroxyl group-directed, tartaric acid-catalyzed synthesis of meta-functionalized aryl ethers and phenols through domino conjugate addition/aromatization of para-quinols†
Abstract
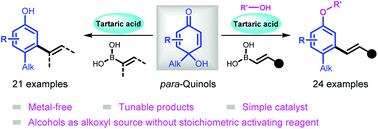
A tartaric acid-catalyzed three-component reaction of para-quinols, organoboronic acids, and alcohols is reported, leading to meta-alkenylated aryl alkyl ether in a single step. Moreover, meta-functionalized phenols could also be obtained in the absence of alcohols. Initial mechanistic studies indicated that the free hydroxy group of p-quinol plays a vital role in initiating the conjugate addition.


 Please wait while we load your content...
Please wait while we load your content...