Highly regio- and stereo-selective heterogeneous 1,3-diyne hydrosilylation controlled by a nickel-metalated porous organic polymer†
Abstract
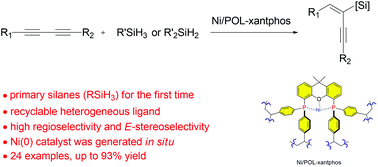
A porous organic polymer (POL-xantphos) was synthesized and employed as a heterogeneous ligand for nickel catalyzed highly regio- and stereo-selective 1,3-diyne hydrosilylation. A broad range of unsymmetrical and symmetrical 1,3-diynes can react with primary and secondary silanes to yield the corresponding silyl-functionalized 1,3-enynes. Owing to the confinement effect of the microporous structure, the selectivity of POL-xantphos greatly increased compared with that of the monomeric xantphos ligand. This is the first report on the use of a porous organic polymer as a regioselective and efficient heterogeneous ligand in 1,3-diyne hydrosilylation. The Earth-abundant base-metal catalyst, coordinated with a recyclable heterogeneous porous organic polymer, shows good prospects for industrial applications.


 Please wait while we load your content...
Please wait while we load your content...