Alkali-regulated Fe6 and Fe18 molecular clusters and their structural transformation†
Abstract
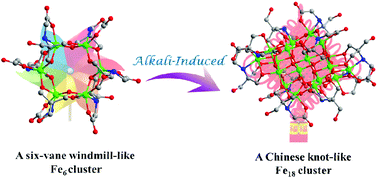
As a kind of metastable aggregation between iron ions and iron hydroxide precipitates, Fe clusters can only be isolated under narrow synthesis conditions. Here, two Fe clusters with different structural models, [Fe6(H2thmmg)6] (Fe6) and [Fe18O8(OH)8(H2thmmg)10] (Fe18), are constructed based on a rare N-tris(hydroxymethyl)methylglycine (H5thmmg) ligand. The cyclic Fe6 can be visualized as a “six-vane windmill” comprised of 6 Fe3+ and 6 H2thmmg3− ligands. Unlike the cyclic configuration of Fe6, the Fe18 cluster features a bricky structure like a “Chinese knot” coordinated by three species, H2thmmg3−, OH− and O2−. It was obtained by adding more triethylamine to the reactive solution of Fe6 which yielded more coordinated species OH− and O2−. Moreover, the conversion from Fe6 to Fe18 was successfully achieved by adding more triethylamine, realizing nuclearity enlargement and structural regulation of Fe clusters. Furthermore, the magnetic properties of Fe6 and Fe18 exhibit antiferromagnetism. This work reports two novel alkali-regulated Fe clusters and further realizes the transformation of nuclearity and structure, which provides some reference for the structural predesign and adjustment of metal nanoclusters.
- This article is part of the themed collection: 2021 Inorganic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...