Application of 4-pyridylselenolate palladium macrocycles in Suzuki couplings†
Abstract
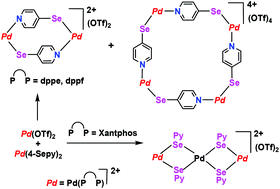
Pd complexes [Pd(P∩P)(4-Sepy)2] of 4-pyridylselenolate (4-Sepy) and diphosphines (P∩P = dppe (1,2-bis(diphenylphosphino)ethane), dppf (1,1′-bis(diphenylphosphino)ferrocene) and Xantphos (4,5-bis(diphenylphosphino)-9,9-dimethylxanthene)) have been developed as metallo-ligands. Their self-assembly reaction with [Pd(P∩P)(OTf)2] (OTf− = CF3SO3−) successfully yielded the cationic macrocyclic complexes [Pd(P∩P)(4-Sepy)]n(OTf)n. The major and minor products are assigned to the dimer (n = 2) and tetramer (n = 4) by nuclear magnetic resonance spectroscopy, electrospray ionization mass spectrometry and X-ray structural analysis. The equilibrium between the dimer and tetramer has been established in compounds of wide-bite-angle dppf. The same reaction for Xantphos as diphosphine produced a selenolato-bridged trinuclear complex with a free pyridyl group. A similar reaction with analogous 4-mercaptopyridine resulted in a mononuclear complex of Xantphos comprising Pd–O bond. Density functional theory calculations establish the stability of the macrocycles as tetranuclear (dppf) > dinuclear (dppf) > dinuclear (dppe). A low energy charge transfer transition has been identified from Se or Fe centers to unoccupied orbitals of mixed character. These macrocycles represent the first examples of metallo-supramolecular complexes constructed from selenolate groups and square-planar metal ions. The Pd complexes showed excellent catalytic activity with high turnover numbers (5 × 107) in Suzuki C–C cross coupling reactions.


 Please wait while we load your content...
Please wait while we load your content...