A zinc/PyBisulidine catalyzed asymmetric Mannich reaction of N-tosyl imines with 3-acyloxy-2-oxindoles†
Abstract
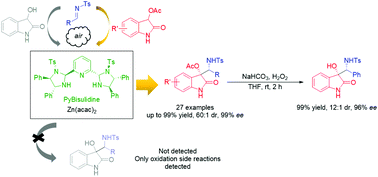
A Zn–PyBisulidine catalyzed asymmetric Mannich reaction of 3-acyloxy-2-oxindoles has been developed. Various quaternary substituted 3-acyloxy-2-oxindoles bearing vicinal amino alcohol motifs were obtained in good to excellent yields with moderate to excellent dr and excellent enantioselectivities. The utility of this reaction was demonstrated by the easy removal of the acyl group to give C3-hydroxy derivatives and their application as a key skeleton of the ligand for the Ni-catalyzed enantioselective Henry reaction.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...