Structure evolution and energy storage mechanism of Zn3V3O8 spinel in aqueous zinc batteries†
Abstract
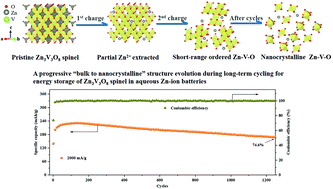
Spinel-type materials are promising for the cathodes in rechargeable aqueous zinc batteries. Herein, Zn3V3O8 is synthesized via a simple solid-state reaction method. By tuning the Zn(CF3SO3)2 concentration in electrolytes and the cell voltage ranges, improved electrochemical performance of Zn3V3O8 can be achieved. The optimized test conditions give rise to progressive structure evolution from bulk to nano-crystalline spinel, which leads to capacity activation in the first few cycles and stable cycling performance afterward. Furthermore, the energy storage mechanism in this nano-crystalline spinel is interpreted as the co-intercalation of zinc ions and protons with some water. This work provides a new viewpoint of the structure evolution and correlated energy storage mechanism in spinel-type host materials, which would benefit the design and development of next-generation batteries.


 Please wait while we load your content...
Please wait while we load your content...