Subtle structure matters: boosting surface-directed photoelectron transfer via the introduction of specific monovalent oxygen vacancies in TiO2†
Abstract
Oxygen vacancies (Ov) are widely considered to play crucial roles in photocatalysis, but how and why they contribute to improved performances remains controversial. In this work, we studied the promotional effect of Ov on photoelectron transfer in TiO2, using first-principles density functional theory calculations with correction for on-site Coulomb interactions. We explicitly identified three types of Ov with different charge states (i.e., charge-neutral 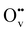 , monovalent
, monovalent 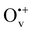 , divalent Ov2+) via electronic structure analysis. Electron transfer energy calculations revealed that the ionized Ov in anatase TiO2 are able to collect excess electrons whereas those in the rutile phase are not. The presence of ionized Ov further endows anatase TiO2 with directional electron transfer along the [100] orientation, in favor of anatase TiO2(101) for photocatalytic reduction surpassing the (001) termination. After examining various combination modes of ionized Ov involving different charge states and spatial distributions, we demonstrated that the vertical
, divalent Ov2+) via electronic structure analysis. Electron transfer energy calculations revealed that the ionized Ov in anatase TiO2 are able to collect excess electrons whereas those in the rutile phase are not. The presence of ionized Ov further endows anatase TiO2 with directional electron transfer along the [100] orientation, in favor of anatase TiO2(101) for photocatalytic reduction surpassing the (001) termination. After examining various combination modes of ionized Ov involving different charge states and spatial distributions, we demonstrated that the vertical 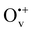 chain in anatase TiO2(101) is the most catalytically effective Ov pattern in TiO2. These results signify the importance of subtle defects in photocatalysis and may assist future photocatalyst design toward higher photocatalytic efficiency.
chain in anatase TiO2(101) is the most catalytically effective Ov pattern in TiO2. These results signify the importance of subtle defects in photocatalysis and may assist future photocatalyst design toward higher photocatalytic efficiency.



 Please wait while we load your content...
Please wait while we load your content...