The second example of doubly enantiophobic behavior during crystallization: a detailed crystallographic, thermochemical and spectroscopic study†
Abstract
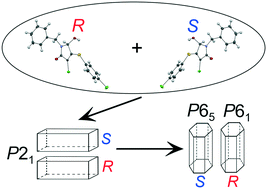
Herein we have found a second example of doubly enantiophobic behavior during the crystallization of rac-1-benzyl-3-chloro-4-[(4-chlorophenyl)sulfanyl]-5-hydroxy-1,5-dihydro-2H-pyrrol-2-one, i.e., the formation of two conglomerates with different crystal structures in the absence of any racemic compounds. In both polymorphic modifications, the main supramolecular motif is appeared to be a hydrogen-bonded chain or helix of different symmetry, which is related to an α-hydroxyamide chain synthon. Additional crosslinking of the homochiral chains in the crystals of both crystalline forms, which is realized through weak, noncovalent interactions, can be a probable reason for the reproducibility and stability of the α-hydroxyamide chain synthon and polymorphic diversity registered. It was shown that each of the conglomerates is thermodynamically stable in certain temperature ranges. For the first time, a binary phase diagram for the chiral system complicated by an enantiotropic phase transition is reconstructed based on experimental data, which is characterized by the presence of a thermodynamic stability permutation line between the two racemic conglomerates.
- This article is part of the themed collection: Supramolecular & Polymorphism


 Please wait while we load your content...
Please wait while we load your content...