Probing two PESIN-indocyanine-dye-conjugates: significance of the used fluorophore†
Abstract
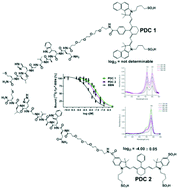
Peptide-dye-conjugates hold a great promise in application for biological and medical imaging of cellular processes and in delineation and characterization of human tumors. In particular, indocyanine dyes are of great interest due to their reported superior properties such as absorption and emission in the near-infrared (NIR) spectral range, favorable Stokes shifts and their well-studied safety profile in humans. In this study, we investigated and describe the influence of indocyanine dyes on different properties of the final peptide-dye-conjugates. As a target peptide, PESIN, a bombesin derivative, was used as a model peptide which addresses GRP receptors overexpressed on different malignancies. Here, we map similarities and differences of the fluorescent conjugates and by this elucidate the influence of the dyes on different properties of the formed conjugates. We performed the dye syntheses, subsequent bioconjugation reactions and in the following investigated the optical properties, water/octanol distribution coefficients and target receptor affinities by in vitro competitive binding studies on PC-3 cells. The obtained results give a handrail to medical and biological researchers planning studies involving indocyanine dye biomolecule conjugates.


 Please wait while we load your content...
Please wait while we load your content...