Cathodic electrochemical deposition: a new strategy to enhance the activity and stability of silver cathodes for thin-film solid oxide fuel cells†
Abstract
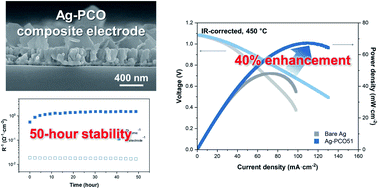
Thin-film-based solid oxide fuel cells with reduced electrolyte thickness have been developed in an effort to lower the operating temperatures and increase the cost competitiveness and lifetime of the devices. Although silver has gained a considerable amount of attention as a cathode material with excellent oxygen reduction activity at lower temperatures, thermal instability due to agglomeration is a major disadvantage associated with the use of silver. Many oxide coating methods have been suggested to overcome this problem, but the results remain unsatisfactory. Here, we propose cathodic electrochemical deposition (CELD) as a new strategy to improve the activity and stability of silver electrodes. Pr-doped ceria is coated onto a nanoporous silver film by CELD and analyzed by electron microscopy, mass spectrometry, and X-ray diffraction. AC impedance spectroscopy of symmetric cells (electrode|electrolyte|electrode) reveals that only a few minutes of CELD increases the electrode activity by approximately 33 times and achieves outstanding durability with no degradation, even at 550 °C for 50 hours. Electrolyte-supported single cells are also fabricated to demonstrate the enhanced performance of the Ag electrode created via the ceria overcoats.


 Please wait while we load your content...
Please wait while we load your content...