Programmable synthesis of multiply arylated cubanes through C–H metalation and arylation†
Abstract
Cubane (C8H8), a cubic alkane, has long attracted attention owing to its unique 3D structure. In order to utilize the cubane scaffold widely in science and technology, a powerful method for synthesizing diverse cubane derivatives is required. Herein, we report the synthesis of mono-, di-, tri-, and tetra-arylated cubanes. Directed ortho-metalation with lithium base/alkyl zinc and subsequent palladium-catalyzed arylation enabled C–H metalation and arylation of cubane. This reaction allows the late-stage and regioselective installation of a wide range of aryl groups, realizing the first programmable synthesis of diverse multiply arylated cubanes.
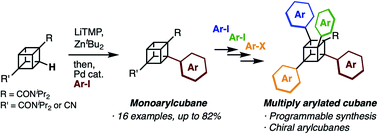
- This article is part of the themed collections: Editor’s Choice – Ning Jiao, Celebrating 10 years of Chemical Science and New reactivity in organic chemistry


 Please wait while we load your content...
Please wait while we load your content...