Balancing interactions in proline-based receptors for chiral recognition of l-/d-DOPA†
Abstract
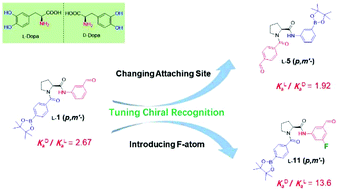
Proline based receptors (1–14) attached with phenylboronic acid and benzaldehyde binding groups at the N-/C- or C-/N-termini of the proline residue were created for chiral recognition of L-/D-DOPA, in an attempt to examine if balancing the two binding events would influence the recognition. By changing the positions of boronic acid and aldehyde groups substituted on the phenyl rings (1–4, 5–8) and the site at which phenylboronic acid and benzaldehyde moieties attached respectively to the N- and C-termini or C- and N-termini of the proline residue (1–4vs.5–8), and by introducing an electron-withdrawing fluorine atom in the phenyl ring of the weaker binder the benzaldehyde moiety (11vs.1, 14vs.5), we were able to show that a better balance of the two binding events does improve the chiral recognition. This finding can only be made with the current version of receptors that were equipped with two different binding groups. Together with the finding that the chiral recognition performance in mixed organic–aqueous solutions is tunable by varying the solvent composition, we have now arrived at a protocol for designing proline based receptors for extended applications in chiral recognition.


 Please wait while we load your content...
Please wait while we load your content...