Photosensitised biphotonic chemistry of pyrimidine derivatives†
Abstract
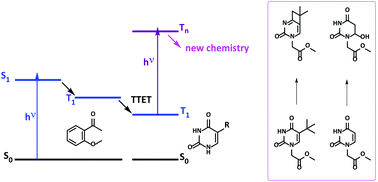
Photosensitised biphotonic irradiation of DNA has been rarely addressed, probably due to the difficulties in the experimental design. This is associated with the selection of nucleobases and sensitisers with appropriate absorption spectra and photochemical reactivity, in combination with a laser source emitting intense UVA light of the adequate wavelength. The present paper presents a new strategy involving absorption of a first UVA photon by an adequate sensitiser followed by triplet energy transfer to a pyrimidine (Pyr) derivative and absorption of a second UVA photon by the resulting Pyr triplet excited state. The feasibility of the proposed strategy has been demonstrated using two model reactions: (i) the Norrish–Yang photocyclisation of a tert-butyluracil and (ii) the photohydration of its uracil analogue, lacking the tert-butyl substituent.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...