Rapid preparation of ultra-fine and well-dispersed SnO2 nanoparticles via a double hydrolysis reaction for lithium storage†
Abstract
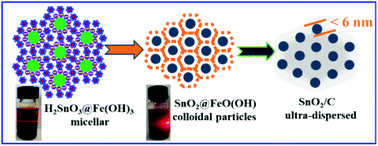
An efficient and rapid method is reported for preparing ultra-fine and well-dispersed SnO2 nanoparticles in a large scale. A simple double hydrolysis reaction between SnO32− and Fe3+ ions was masterly used to form a stable colloid system, in which colloidal particles of H2SnO3 with negative charges and Fe(OH)3 with positive charges electrostatically interact with each other and form honeycomb-like “core–shell” units. Through the hydrothermal reaction, the units are easily transformed into SnO2@FeO(OH) structures. Ultra-fine and well-dispersed SnO2 particles with less than 6 nm diameter were finally obtained with a high yield by further etching using hydrochloric acid. When used as anode materials for lithium ion batteries, the ultra-fine SnO2 particles can be easily dispersed into the carbon networks originating from the carbon source of glucose during the hydrothermal reaction. Electrochemical tests confirmed that these ultra-fine SnO2/C materials were endowed with excellent cyclic stability and C-rate performance. Even at a 1.56 A g−1 (2C) high current density, the reversible capacity could be maintained at 710 mA h g−1 after 100 cycles owing to the ultra-fine particle size of SnO2 and the rich carbon networks.


 Please wait while we load your content...
Please wait while we load your content...