Synthesis and structural characterisation of bulky heptaaromatic (hetero)aryl o-substituted s-aryltetrazines†
Abstract
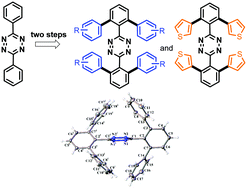
An expedient two-step synthesis produces in good yield polyaromatic heptacyclic (hetero)arylated o-substituted s-aryltetrazines (s-Tz) directly from diphenyl s-tetrazine. This methodology overcomes the steric limitations of classical Pinner-like syntheses encountered for o-functionalized s-Tz. A single step palladium-catalyzed N-directed C–H bond tetrahalogenation is followed by a Pd-catalyzed Suzuki (hetero)arylation that is achieved simultaneously on four sites. The single crystal X-ray diffraction structure of the resulting typical polyaromatic heptacyclic aromatic compound 3,6-bis(2,6-diphenyl)-1,2,4,5-tetrazine (3) is analyzed, together with R-functionalized peripheral phenyl derivatives [R = p-t-Bu (4), and m-OMe (10)]. Generally, stacking interactions between aromatic rings can be considerably stronger if electron-depleted rings are combined with electron-rich ones. Thus, electron-poor heteroaromatic aryltetrazines are expected to interact with electron-rich phenyl aromatic rings from reduced π⋯π repulsion, rendering the formation of stacking networks more favorable. Herein, despite the presence of strongly electron-deficient heteroaromatic tetrazine cores, the disruption of planarity between the various aromatic rings involved precludes the expected stacking arrangement. Thus, packing organization is driven by weak hydrogen bonding with C–H⋯N short contacts (or C–H⋯O when possible). These new heptaaromatics, which incorporate for the first time an electron-attracting nitrogen-rich core are easily modifiable from cross-coupling reactions, and constitute a relevant structural and electronical alternative to the well-known and high value class of hexaphenylbenzenes.


 Please wait while we load your content...
Please wait while we load your content...