Alkaloids and lignans with acetylcholinesterase inhibitory activity from the flower buds of Magnolia biondii Pamp†
Abstract
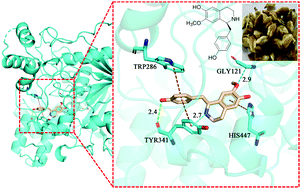
Two previously undescribed alkaloids, 4,4′-dihydroxy-3-methoxy-paucine-4′-O-β-D-glucopyranoside and (S)-2-(1,3-propanediol-2-yl)-isococlaurine, and three new lignans, (7R,8S)-9,9′-dihydroxy-3,4-dimethoxy-7,8-dihydrobenzofuran lignan, (7R,8S)-4,9,9′-trihydroxy-3,3′-dimethoxy-9′-acetoxy-7,8-dihydrobenzofuran lignan-4-O-β-D-glucopyranoside, and (7S,8R)-4,9,9′-trihydroxy-3,3′,5-trimethoxy-9′-acetoxy-7,8-dihydro-benzofuran lignan-4-O-β-D-glucopyranoside, together with eleven known compounds, were isolated from the flower buds of Magnolia biondii Pamp. Their structures were determined by extensive spectroscopic analysis, and their absolute configurations were deduced by analysis of optical rotation and electronic circular dichroic (ECD) spectra. All the isolated compounds were evaluated in vitro for their acetylcholinesterase (AChE) inhibitory activity. As a result, it was found that (+)-isococlaurine exhibited the strongest AChE inhibitory effect. Molecular docking experiments were carried out to identify the probable binding mode of this compound in the binding sites of AChE and molecular dynamics simulations were performed to evaluate the stability over time of the main interactions observed during docking calculations.


 Please wait while we load your content...
Please wait while we load your content...