Neutrophilic infiltration in organ-on-a-chip model of tissue inflammation†
Abstract
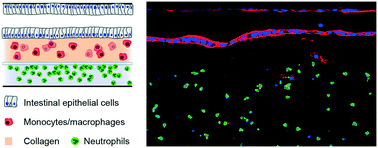
The multiphasic etiology of tissue inflammation and the fundamental immunological differences between species render inflammatory pathologies difficult to recapitulate in animal models, and account for the paucity of therapies that are successfully translated from rodents to humans. Here, we present a human-relevant organ-on-a-chip platform for experimental inflammatory diseases. We created an immunocompetent in vitro gut model by incorporating intestinal epithelial and immune cells into microfluidic chambers that permit cell movement across an extracellular matrix (ECM) and fluidic channels. This is the first model that integrates a mucosal barrier, a three-dimensional ECM, resident and infiltrating immune cells, and simulates a functional crosstalk that ultimately triggers cellular processes representative of inflammation. Under homeostatic conditions, enterocytes form a tight epithelium and subepithelial macrophages are non-activated. Introduction of pro-inflammatory mediators triggers macrophage activation and inflammation-induced intestinal barrier leakiness. Neutrophils in a parallel, matrix-separated non-epithelial channel are attracted by such a pro-inflammatory microenvironment and migrate through the extracellular matrix, further exacerbating tissue inflammation and damage. With this model, we provide the foundations to recapitulate and investigate the onset of tissue inflammation in a controlled, human-relevant system.


 Please wait while we load your content...
Please wait while we load your content...