Structure–property relationships in multi-stimuli responsive BODIPY-biphenyl-benzodithiophene TICT rigidochromic rotors exhibiting (pseudo-)Stokes shifts up to 221 nm†
Abstract
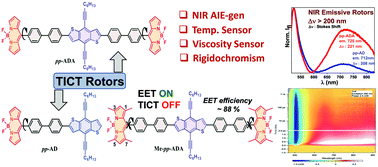
Structure–property relationships of donor–π–acceptor (D–π–A) type molecular dyad (pp-AD) and triads (pp-ADA and Me-pp-ADA) based on benzodithiophene and BODIPY with biphenyl spacers have been reported. Rotors pp-AD and pp-ADA showed efficient twisted intramolecular charge transfer (TICT) with near infrared (NIR) emissions at ∼712 nm and ∼725 nm with (pseudo-)Stokes shifts of ∼208 nm and ∼221 nm, respectively, and prominent solvatochromism. A structurally similar triad, Me-pp-ADA, with tetramethyl substituents on the BODIPY core instead was TICT inactive and exhibited excitation energy transfer with a transfer efficiency of ∼88% as revealed using steady state emission and transient absorption measurements. Rotors pp-AD and pp-ADA showed NIR emission with an enhancement in intensity with the addition of water in THF solution as well as a pronounced change in emission intensity with temperature and viscosity variations, which justify their utility as temperature and viscosity sensors. Furthermore, the linear correlation of lifetime with fluorescence intensity ratios of the donor and acceptor justifies the rigidochromic behaviour of these rotors.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...