Porous nickel electrodes with controlled texture for the hydrogen evolution reaction and sodium borohydride electrooxidation†
Abstract
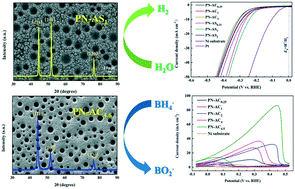
Porous Ni electrodes with different textures have been fabricated by a facile electro-deposition method. The additives ammonium chloride (NH4Cl) and ammonium sulfate ((NH4)2SO4) in supporting electrolytes play a vital role in the texture of porous electrodes. Porous Ni electrodes prepared with the NH4Cl additive present a (111) plane preferred orientation. However, with the additive (NH4)2SO4, as its concentration increases, the preferred orientation changes from the (111) plane to the (200) plane. In addition, we studied the texture of the porous Ni electrodes and their influence on the hydrogen evolution reaction (HER) and sodium borohydride (NaBH4) electrooxidation. Porous Ni electrodes with the (111) preferential orientation promote the electrooxidation of NaBH4. Meanwhile, optimum catalytic activity can be obtained when both the (111) and (220) planes are preferentially oriented. In contrast, porous Ni electrodes with the (200) preferential orientation facilitate NaBH4 hydrolysis. Interestingly, the porous Ni electrode with both the (200) and (220) preferential orientations exhibits the best HER catalytic activity. This work provides new insights into the relationship between the texture and the electrocatalytic properties of porous Ni electrodes, which is desirable to design nickel electrodes more reasonably for HER or sodium borohydride electrooxidation.


 Please wait while we load your content...
Please wait while we load your content...