Stable Ln-MOFs as multi-responsive photoluminescence sensors for the sensitive sensing of Fe3+, Cr2O72−, and nitrofuran†
Abstract
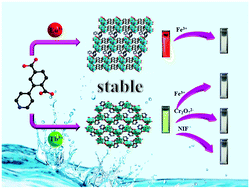
Two families of lanthanide(III) metal–organic frameworks (Ln-MOFs) constructed from a 2-(4-pyridyl)-terephthalic acid (H2pta) ligand, namely [Ln4(pta)5(Hpta)2(H2O)4]·xH2O (1-Eu, 2-Gd) and [Ln(Hpta)(C2O4)]·3H2O (3-Tb, 4-Dy, 5-Er and 6-Ho) (C2O4 = oxalic acid), have been discovered using the solvothermal method. On the basis of thermodiffractometry, all MOFs retain their crystallinity and main structure below 370 °C. Utilizing their excellent water stability and chemical stability, luminescence studies have been carried out. Notably, the Eu-metal organic framework (MOF) and Tb-MOF demonstrate pronounced luminescence, as well as high stability in water and other organic solvents. The luminescence explorations reveal that the Eu-MOF possesses good selectivity for testing Fe3+ in the aqueous phase via fluorescence quenching, while the Tb-MOF behaves as a multifunctional chemical sensor for the detection of Fe3+ cations and Cr2O72− anions in aqueous media and the recognition of nitrofuran (NIF) in DMF solvent. The recyclability and stability of Eu-MOF and Tb-MOF after the sensing experiments were observed to be adequate. By virtue of the superior stability, detection efficiency, applicability and reusability, Eu-MOF and Tb-MOF could be promising fluorescent materials for practical sensing applications.


 Please wait while we load your content...
Please wait while we load your content...