Engineering MoS2 nanostructures from various MoO3 precursors towards hydrogen evolution reaction†
Abstract
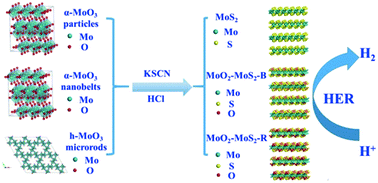
MoS2-based nanomaterials are considered promising effective electrocatalysts to replace precious metal catalysts for hydrogen evolution reaction. However, understanding the effect of MoO2 in MoS2-based catalysts for hydrogen evolution reaction is ambiguous. In this paper, MoS2 nanoflowers of 210–430 nm in diameter were hydrothermally synthesized by the reduction of α-MoO3 particles with KSCN in a hydrochloric acid medium. Similarly, MoO2–MoS2-B nanoflowers and MoO2–MoS2-R nanoflowers were fabricated using α-MoO3 nanobelts and h-MoO3 microrods as Mo sources, respectively. Systematic studies on synthetic parameters verified that the formation of MoS2 nanoflowers is favored with high acidity, low MoO3/KSCN ratio and high temperature. The resultant nanoflowers served as electrocatalysts to drive hydrogen evolution in acidic solution. The MoS2 nanoflowers showed a relatively higher activity with a potential of 256 mV at 10 mA cm−2 compared to MoO2–MoS2-B nanoflowers (283 mV) and MoO2–MoS2-R nanoflowers (305 mV). Furthermore, MoS2 nanoflower catalysts maintained high stability after 1000 cycles and long-term durability for 5 h. The high catalytic activity could be ascribed to the large exposure of the Mo–S species and small amounts of Mo–O species on the MoS2-based catalyst surface.


 Please wait while we load your content...
Please wait while we load your content...