Scope and mechanism of nitrile dihydroboration mediated by a β-diketiminate manganese hydride catalyst†
Abstract
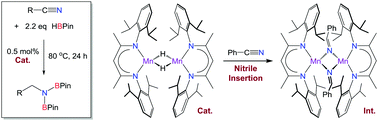
The manganese hydride dimer, [(2,6-iPr2PhBDI)Mn(μ-H)]2, was found to mediate nitrile dihydroboration, rendering it the first manganese catalyst for this transformation. Stoichiometric experiments revealed that benzonitrile insertion affords [(2,6-iPr2PhBDI)Mn(μ-NCHC6H5)]2 en route to N,N-diborylamine formation. Density functional theory calculations reveal the precise mechanism and demonstrate that catalysis is promoted by monomeric species.


 Please wait while we load your content...
Please wait while we load your content...