mTORC1-dependent TFEB nucleus translocation and pro-survival autophagy induced by zeolitic imidazolate framework-8†
Abstract
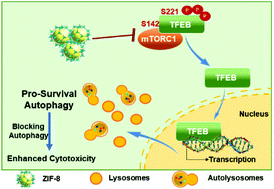
A great variety of nanoparticles are known to induce autophagy, leading to either pro-death or pro-survival. Zeolitic imidazolate framework-8 (ZIF-8), a type of porous metal–organic framework (MOF) material and a promising drug delivery vector, has reportedly shown excellent efficacy for cancer therapy. However, less attention has been paid to the potential biological effect of ZIF-8 per se, and if so, how the effect impacts cell fate and therapy outcomes. Herein, we showed that ZIF-8 induced autophagy in HeLa cells, characterized by increased autophagosome formation without disruption of autophagic flux, in a dose- and time-dependent fashion. ZIF-8 also caused dephosphorylation of the transcription factor EB (TFEB) at serine-142 and serine-211, leading to the nucleus translocation of TFEB, an event that promoted lysosome biogenesis and is necessary for autophagy induction. We further pinpointed the inhibition of mTORC1 as the critical event upstream of ZIF-8-elicited TFEB dephosphorylation and the subsequent nucleus translocation. Furthermore, autophagy induced by ZIF-8 promoted cell survival, as inhibiting autophagy by either 3-methyladenine (3-MA) or ATG5 knockdown significantly enhanced ZIF-8-elicited HeLa cell death. Most importantly, doxorubicin-encapsulated ZIF-8 (DOX@ZIF-8) also elicited strong pro-survival autophagy, and the co-delivery of an autophagic inhibitor (3-MA) dramatically enhanced the cytotoxicity of DOX@ZIF-8 in HeLa cells. Our results revealed the unique ability of ZIF-8, both in a free and drug-loaded form, to induce pro-survival autophagy in certain cancer cells, a finding with important implications for potential clinical studies that utilize ZIF-8 as a drug carrier.


 Please wait while we load your content...
Please wait while we load your content...