Catalytic activity for dissociative oxygen adsorption of Co-based oxides at high temperature evaluated by a modified pulse isotopic exchange technique†
Abstract
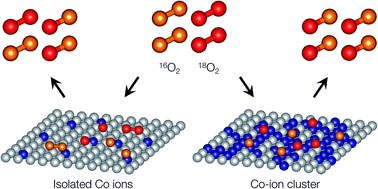
Improving the oxygen surface exchange reaction rate in the intermediate temperature range is one of the most important challenges in the effort to facilitate the widespread use of high temperature electrochemical devices such as solid oxide fuel cells. While the fast surface exchange kinetics of Co-based mixed ionic conductors is well known, it is also believed that Co-based oxides which have negligible oxygen incorporation reactions, such as Co3O4, are superior catalysts for surface-exchange reactions. In this investigation, a modified pulse isotopic exchange technique was used to quantitatively evaluate the dissociative adsorption rate of Co–Fe-based spinel-type and Co–Mg-based rock salt-type oxides. Catalytic activity increased exponentially as the Co concentration increased regardless of the crystal structure. This trend can be explained by site percolation probability, indicating the importance of Co clusters in catalytic activity. The dissociative adsorption rate of Co3O4 was found to be comparable with the overall surface exchange rate of mixed oxide-ion and electronic conductors.


 Please wait while we load your content...
Please wait while we load your content...