Highly porous Ti–Ni anodes for electrochemical oxidations†
Abstract
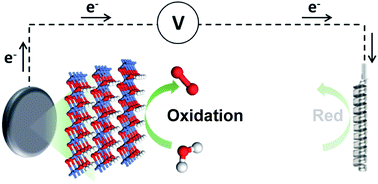
This work describes a general method to obtain highly porous electrodes and their use as dimensionally stable anodes for the O2 evolution reaction (OER). By using a powder metallurgy based process, where metallic titanium and nickel powders are pressed and thermally treated to form “brown compacts”, we obtain electrodes that benefit from a 1000-fold increase in the electrochemical surface area (ECSA). In addition, active catalytic species for water oxidation (i.e. NiOOH) are generated during the processing converting these electrodes as ideal candidates for evaluation in oxidation reactions. Excellent OER performance is obtained with overpotentials below 270 mV at 10 mA cm−2, exceeding those of commercially available alternatives. The current work paves the way for a generic method that will be extended to other electrochemical reactions.


 Please wait while we load your content...
Please wait while we load your content...