Dithioesters: simple, tunable, cysteine-selective H2S donors†
Abstract
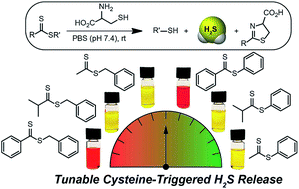
Dithioesters have a rich history in polymer chemistry for RAFT polymerizations and are readily accessible through different synthetic methods. Here we demonstrate that the dithioester functional group is a tunable motif that releases H2S upon reaction with cysteine and that structural and electronic modifications enable the rate of cysteine-mediated H2S release to be modified. In addition, we use (bis)phenyl dithioester to carry out kinetic and mechanistic investigations, which demonstrate that the initial attack by cysteine is the rate-limiting step of the reaction. These insights are further supported by complementary DFT calculations. We anticipate that the results from these investigations will allow for the further development of dithioesters as important chemical motifs for studying H2S chemical biology.


 Please wait while we load your content...
Please wait while we load your content...