Enhanced CO2 electroreduction via interaction of dangling S bonds and Co sites in cobalt phthalocyanine/ZnIn2S4 hybrids†
Abstract
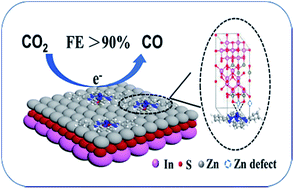
The efficient electrochemical reduction of CO2 to CO in aqueous electrolyte is very interesting. Due to the critical electron-transfer step during the activation of CO2, it is important to design efficient strategies to engineer the electronic properties of catalysts to improve the electrochemical performance. Herein cobalt phthalocyanine (CoPc) supported on ZnIn2S4 (ZIS) nanosheets was synthesized. It was found that the hybrids showed excellent performance for CO2 electroreduction to CO in aqueous solution. The faradaic efficiency, current density and mass activity could reach 93%, 8 mA cm−2 and 266 mA mg(CoPc)−1, respectively. Introduction of Zn-defects resulted in dangling S bonds in the ZIS support, which interacted with Co active sites of CoPc via strong Co–S interaction. Mechanistic studies revealed that the enhancement of CO production over CoPc by Co–S interaction originated from the eased CO2 activation.


 Please wait while we load your content...
Please wait while we load your content...